PLATFORM OVERVIEW
Hyper-Accelerate Digitalization of Pharma
InSilicoTrials is a unique simulation platform that cuts the cost of R&D and accelerates regulatory approval for drugs
PLATFORM OVERVIEW
InSilicoTrials is a unique simulation platform that cuts the cost of R&D and accelerates regulatory approval for drugs
Crowdscience
We build a bridge between science and industry by integrating validated and regulatory compliant models
Scalable
Flexible computational power, a collection of models and AI tools in a pay-per-use mode. No software or fixed costs
User Friendly
Simplified user interfaces and workflows allow also professionals with no computational expertise to set up and run complex simulations
In today’s fast-paced business landscape, Medical Technology companies must prioritize de-risking innovation and increasing efficiency to stay competitive. One way to achieve these goals is by embracing digitalization, particularly in the area of R&D.
Digitalization can streamline processes, reduce costs and enhance collaboration, ultimately leading to more effective innovation and long-term success.
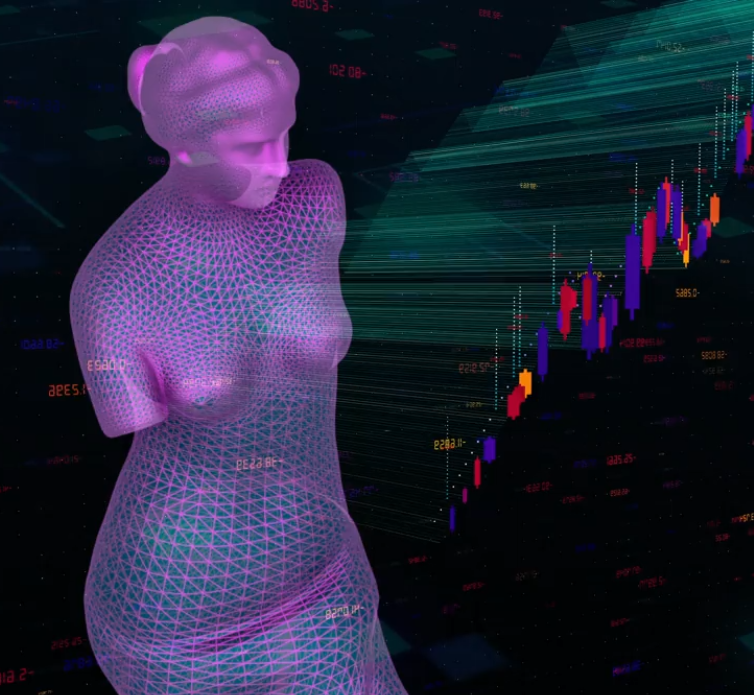
Buy tokens and use the products of your choice among those available on the platform
We can build simulation-based products and create AI tools for healthcare, according to the specific requests of our clients, making them available as SaaS
We help your company leverage the best technologies available today
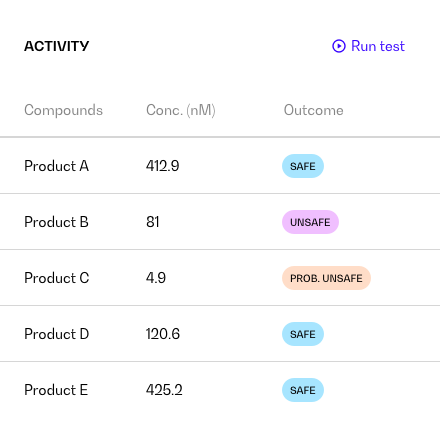
Upload your own data and set parameters
Run the simulation and get your outcomes
Download your report in compliance with regulatory’s requirements
Our cloud platform guarantees the highest level of data security. Our digital products are ready to use, so our clients can reduce all costs related to very expensive software, IT infrastructures, and specialized personnel.
[formidable id=”14″]